We routinely write about phony chemical scares. Thanks to know-nothings, groups with agendas, and the press, the mere mention of BPA, which is about as close to harmless as chemicals get, sends people into a frenzy. Some will go to great lengths to avoid it, such as refusing to touch cash register receipts.
But, there is nothing phony about phosphine—the gas that recently killed four children in Texas. Phosphine is a real poison and a very potent one at that. Most chemists will go their entire careers without ever using it.
This accident provides an unfortunate opportunity to examine the chemistry of what happened.
Tragically, and inadvertently, a father, who had spread aluminum phosphide under his home to kill insects, caused the accident by trying to wash it away. The culprit was the water.
The reaction that caused the fumigant aluminum phosphide to turn into deadly phosphine is one of the most elementary in chemistry—the acid-base reaction, also known as neutralization.
A base is a chemical that, when dissolved in water will result in the pH rising above 7 (on a scale from 0-14). The higher the pH, the more basic the solution (1), and the more dangerous it becomes. Bases turn pH paper blue; acids turn them red:
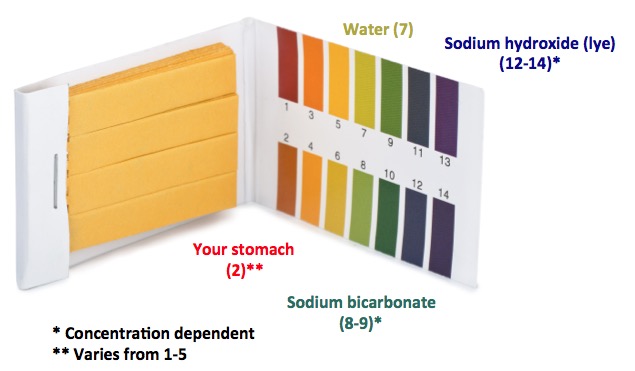
Litmus paper colors depending on pH. Original photo: Shutterstock
The following chemical reactions demonstrate a reason why aluminum phosphide is used as a pesticide, (top) and the cause of the accident (bottom).
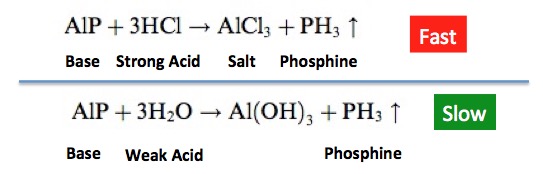
Aluminum phosphide reacts very quickly under acidic conditions—such as those in the stomach of a rat—and liberates phosphine, a very deadly gas (the arrows to the right of the equation means that a gas is formed). The phosphine kills the rat (2). But water, even though it is a very weak acid, will react with the chemical, albeit slowly, to also produce phosphine. This is why every warning label on the product tells you to keep it dry.
Although phosphine is highly toxic, it is nonetheless used widely (and usually safely) because of one property—its boiling point. It turns from a liquid to a gas at -126 degrees F—well below even the coldest place on earth, so once it forms, it evaporates immediately. You just have to stay away long enough.
Chemistry can be very strange, and also deadly. Who could imagine that a garden hose could cause such a tragedy?
Notes:
(1) Sodium hydroxide (lye) is extremely basic. Bases react with and break down fats and oils. This is why it is used as a drain opener.
(2) Phosphine kills insects by a different mechanism
No comments:
Post a Comment