Whether it's in the body or in a lab, chemical reactions are omnipresent; life depends on thousands of chemical reactions as do virtually all of the products that we use every day.
The concept is straightforward. A chemical reaction is a process in which a chemical compound (a reactant) is converted into a different compound (a product). The product of a chemical reaction is not only an entirely different molecular entity but also will usually bear no resemblance whatsoever to any of the reactant(s). No chemical reaction demonstrates this better than what happens when sodium and chlorine are combined (Figure 1).
1. Two deadly chemicals can react to form one that is harmless
Sodium is a soft, highly reactive metal, which explodes when added to water. If you put it in your mouth your head will explode. Chlorine is a deadly green gas, which was one of the first chemical weapons ever used (e.g., in World War I). Even a single whiff can be deadly. Sodium chloride clearly retains no properties of the two reactants nor does it revert to either. Two deadly chemicals form one harmless one.

Figure 1. Explosive sodium and deadly chlorine react to give table salt. Source: Saddlespace
2. A harmless reactant can form a toxic product
Figure 1 (above) showed an example of a chemical reaction that made two deadly chemical safe. But it also works the other way around (Figure 2).
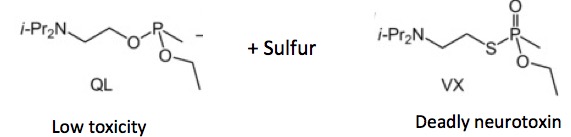 Figure 2. The chemical reaction that produces VX gas.
Figure 2. The chemical reaction that produces VX gas.
O-(2-diisopropylaminoethyl) O'-ethyl methylphosphonite, which is mercifully abbreviated QL is the synthetic precursor (1) to VX gas (2), one of the worst toxins around. QL has very little toxicity. But when you combine it with sulfur and heat the reaction, look out. It is unlikely that you will make it out of the lab alive. Ten milligrams, less than one drop, is fatal when applied to the skin.
3. Neutralizing the toxin you just made
Chemical reactions can neutralize dangerous chemicals or make them. When used in sequence, they can do both. VX was made by reaction with two low-toxicity chemicals. But when deadly VX is reacted with sodium hydroxide all the toxicity "goes away" (Figure 3).
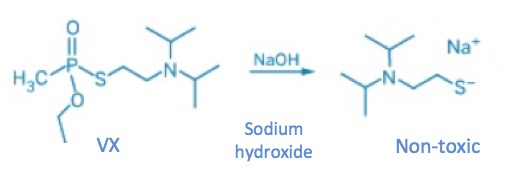 Figure 3. Neutralization of VX with sodium hydroxide Source: Chemical and Engineering News
Figure 3. Neutralization of VX with sodium hydroxide Source: Chemical and Engineering News
The underlying message here is the essence of chemistry: It doesn't matter how a chemical is made.
Every chemical compound has its own set of physical, chemical and toxicological properties. So when The Food Babe claims that Subway bread is unsafe because it is made with a chemical that is also used to make rubber yoga mats, it is a meaningless statement, something she should know.
Now you do.
1. Two deadly chemicals can react to form one that is harmless
Sodium is a soft, highly reactive metal, which explodes when added to water. If you put it in your mouth your head will explode. Chlorine is a deadly green gas, which was one of the first chemical weapons ever used (e.g., in World War I). Even a single whiff can be deadly. Sodium chloride clearly retains no properties of the two reactants nor does it revert to either. Two deadly chemicals form one harmless one.

Figure 1. Explosive sodium and deadly chlorine react to give table salt. Source: Saddlespace
2. A harmless reactant can form a toxic product
Figure 1 (above) showed an example of a chemical reaction that made two deadly chemical safe. But it also works the other way around (Figure 2).
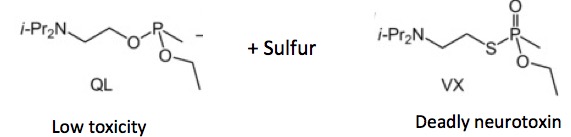
O-(2-diisopropylaminoethyl) O'-ethyl methylphosphonite, which is mercifully abbreviated QL is the synthetic precursor (1) to VX gas (2), one of the worst toxins around. QL has very little toxicity. But when you combine it with sulfur and heat the reaction, look out. It is unlikely that you will make it out of the lab alive. Ten milligrams, less than one drop, is fatal when applied to the skin.
3. Neutralizing the toxin you just made
Chemical reactions can neutralize dangerous chemicals or make them. When used in sequence, they can do both. VX was made by reaction with two low-toxicity chemicals. But when deadly VX is reacted with sodium hydroxide all the toxicity "goes away" (Figure 3).

The underlying message here is the essence of chemistry: It doesn't matter how a chemical is made.
Every chemical compound has its own set of physical, chemical and toxicological properties. So when The Food Babe claims that Subway bread is unsafe because it is made with a chemical that is also used to make rubber yoga mats, it is a meaningless statement, something she should know.
Now you do.

No comments:
Post a Comment